Next-Generation Sequencing Services
ElevateBio has a wide range of sequencing options to serve all cell and gene therapy modalities.


// Manufacturing &
Development Services
Rapid Next-Generation Sequencing Services are Designed to Support all Cell and Gene Therapy Modalities
ElevateBio’s next-generation sequencing (NGS) leverages advanced technologies and expertise to provide flexibility, customizability, and rapid turnaround times.

Adriana Geldart
Senior Director, Next-Generation Sequencing
We are Enabling Industry Partners with our NGS services
Instrumentation & CapabilitiesWe have an ever-evolving range of next-generation sequencing capabilities with integrated bioinformatics analysis and robust instrumentation including Illumina, Oxford Nanopore, and 10x Genomics. |  |
Data QualityOur end-to-end processes—from sample recording to preparation and data analysis—are powered by digital-native automated pipelines and laboratory automation, designed for scalability and high-throughput efficiency. We achieve requisite coverage without compromising accuracy or quality through optimized protocols, assay controls, and automated bioinformatics. This integration leads to faster turnaround, with workflows reduced to <1 week and data for advanced assays delivered 2-4 weeks faster than industry standard. |  |
ApplicationsWe offer a range of sequencing solutions, including amplicon, whole exome, whole genome, RNA (including single-cell), methyl, and complete plasmid. | 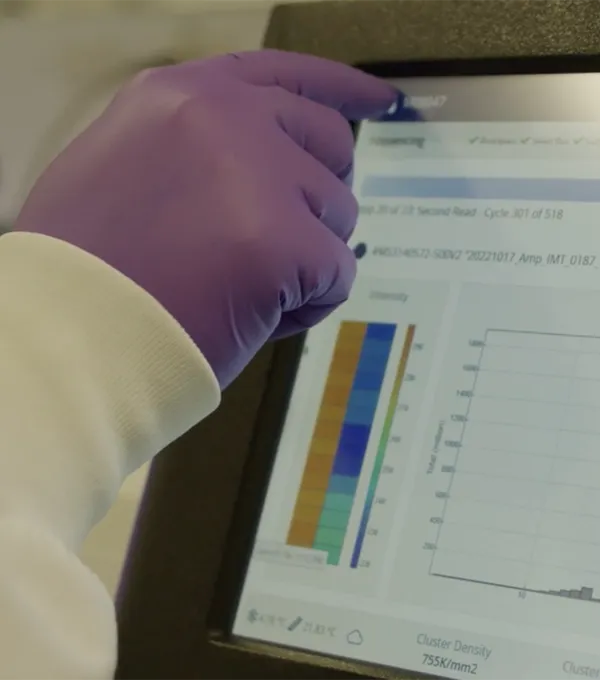 |

Why FDA Complete Response Letters Involve Manufacturing Issues – and What Must Change in Cell Therapy
By Cindy Riggins, Head of CMC Regulatory Affairs, ElevateBio
Elevated Insights
From gene editing to biomanufacturing, learn more about the cell and gene therapy industry from our team of industry-leading experts.
How does next-generation sequencing work?

Partner with ElevateBio®
Wherever you are in your product lifecycle, we can strengthen and accelerate the development of your transformative therapies with our enabling technologies unmatched manufacturing capabilities.
// Work with us
